Transport and industry decarbonisation
Some industrial processes, like the making of steel, aluminium, cement and fertiliser, require very high temperatures, produce greenhouse gas emissions as a by-product of chemical processes or use fossil fuels as feedstocks. This means they are difficult to decarbonise.
Transport is also difficult to decarbonise due to growing global demand and dependence on fossil fuels. For example, in aviation with current technology, a battery that could store enough energy for a mid to long-range flight would be too heavy and bulky.
Yet, to transition to net zero emissions, we need to capture billions of tonnes of CO2 from the source point and the atmosphere.
Grabbing emissions from the air
CSIRO is developing ways to achieve cost-effective and efficient capture of CO2 directly from the air, but there are challenges. The process requires a large amount of energy, and the equipment and capture materials can be costly.
The Ambient CO2 Harvester
ACOHA is the Ambient CO2 Harvester, developed by CSIRO researchers for direct air capture of CO2.
The air capture process requires lots of energy, making it expensive. This is an important obstacle to further development and large-scale deployment. The low concentration of CO2 also means that large volumes of air need to be treated to capture it. This means we need large devices, which can be expensive.
Our interdisciplinary team, including mechanical and chemical engineers, chemists and biologists, is focused on reducing costs in the development of the process, by using a non-volatile capture agent so that a wash column is no longer needed, and a less expensive off-the-shelf cooling tower equipment instead of a stainless-steel packed column.
Other innovations in the process included the formulation and selection of suitable liquid absorbents and more efficient ways to regenerate them.
Absorbents to soak up liquid and gas
Absorbents are substances that absorb or 'soak up' liquid or gas easily. The most efficient absorbents in the capture process can capture a large amount of CO2 quickly, and then need the least amount of energy to be regenerated afterwards when the CO2 is released.
This regeneration accounts for a large part of the energy costs of CO2 capture.
We tested a range of novel liquid absorbents, ionic liquids, solid absorbents and enzymes for capacity and speed of absorption and for energy use during reagent regeneration. This helped us focus on amino-acid-based absorbents which are stable and perform well, with a high capacity for cycling between absorption and desorption.
To store or use
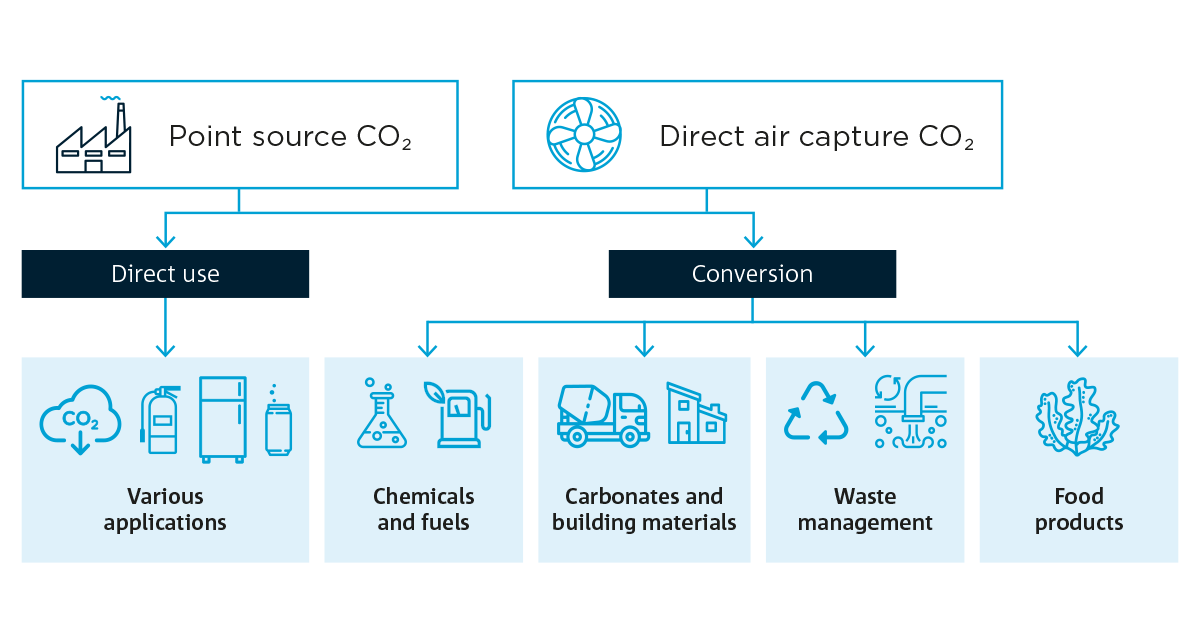
What happens next? Once the CO2 is captured, it can be released and compressed, transported, and injected into a porous rock reservoir capped with impermeable rock to stop the gas from moving upwards. This is called geosequestration, a long-term storage solution that removes the CO2 from the atmosphere completely.
We can also re-use the CO2 directly in refrigeration or to carbonate our drinks or, after conversion, into chemicals and fuels, building materials, food products and more.
The CO2 Utilisation Roadmap details how we can use the CO2 we capture to create new revenue streams and cost-competitive products.
More information
CSIRO has a strong track record of working with government, industry and research organisations. Find out more about our research, development and innovation in CO2 capture, use and storage:
- carbon dioxide removal research, the CarbonLock Future Science Platform
- ACOHA, the Ambient CO2 Harvester
- using tiny crystal sponges to capture and recycle CO2 with Airthena™
- CarbonAssist, a small and inexpensive unit for capturing CO2 that's ready to deploy.
Transport and industry decarbonisation
Some industrial processes, like the making of steel, aluminium, cement and fertiliser, require very high temperatures, produce greenhouse gas emissions as a by-product of chemical processes or use fossil fuels as feedstocks. This means they are difficult to decarbonise.
Transport is also difficult to decarbonise due to growing global demand and dependence on fossil fuels. For example, in aviation with current technology, a battery that could store enough energy for a mid to long-range flight would be too heavy and bulky.
Yet, to transition to net zero emissions, we need to capture billions of tonnes of CO2 from the source point and the atmosphere.
Grabbing emissions from the air
CSIRO is developing ways to achieve cost-effective and efficient capture of CO2 directly from the air, but there are challenges. The process requires a large amount of energy, and the equipment and capture materials can be costly.
The Ambient CO2 Harvester
ACOHA is the Ambient CO2 Harvester, developed by CSIRO researchers for direct air capture of CO2.
The air capture process requires lots of energy, making it expensive. This is an important obstacle to further development and large-scale deployment. The low concentration of CO2 also means that large volumes of air need to be treated to capture it. This means we need large devices, which can be expensive.
Our interdisciplinary team, including mechanical and chemical engineers, chemists and biologists, is focused on reducing costs in the development of the process, by using a non-volatile capture agent so that a wash column is no longer needed, and a less expensive off-the-shelf cooling tower equipment instead of a stainless-steel packed column.
Other innovations in the process included the formulation and selection of suitable liquid absorbents and more efficient ways to regenerate them.
Absorbents to soak up liquid and gas
Absorbents are substances that absorb or 'soak up' liquid or gas easily. The most efficient absorbents in the capture process can capture a large amount of CO2 quickly, and then need the least amount of energy to be regenerated afterwards when the CO2 is released.
This regeneration accounts for a large part of the energy costs of CO2 capture.
We tested a range of novel liquid absorbents, ionic liquids, solid absorbents and enzymes for capacity and speed of absorption and for energy use during reagent regeneration. This helped us focus on amino-acid-based absorbents which are stable and perform well, with a high capacity for cycling between absorption and desorption.
To store or use
Flow diagram showing potential direct or indirect use of carbon dioxide in a variety of applications and products.
What happens next? Once the CO2 is captured, it can be released and compressed, transported, and injected into a porous rock reservoir capped with impermeable rock to stop the gas from moving upwards. This is called geosequestration, a long-term storage solution that removes the CO2 from the atmosphere completely.
We can also re-use the CO2 directly in refrigeration or to carbonate our drinks or, after conversion, into chemicals and fuels, building materials, food products and more.
The CO2 Utilisation Roadmap details how we can use the CO2 we capture to create new revenue streams and cost-competitive products.
More information
CSIRO has a strong track record of working with government, industry and research organisations. Find out more about our research, development and innovation in CO2 capture, use and storage:
- carbon dioxide removal research, the CarbonLock Future Science Platform
- ACOHA, the Ambient CO2 Harvester
- using tiny crystal sponges to capture and recycle CO2 with Airthena™
- CarbonAssist, a small and inexpensive unit for capturing CO2 that's ready to deploy.
