Australia’s iron ore makes a significant contribution to our economy, with a gross added value of over $100 billion each year. However the process of turning the world’s iron ore into steel currently contributes around seven per cent of global greenhouse gas emissions. There are a number of ways of reducing the carbon footprint of the iron making industry, one of the most topical methods being the use of renewable hydrogen in the process.
How iron is made
Once iron ore is removed from the earth, it must be crushed into small pieces and then smelted to remove most of the oxygen, carbon and other impurities. The smelting process produces ‘pig iron’ which can be used to make cast iron, but most of it is converted into steel. Here is a simplified view of the current process, with inputs and outputs for each step:
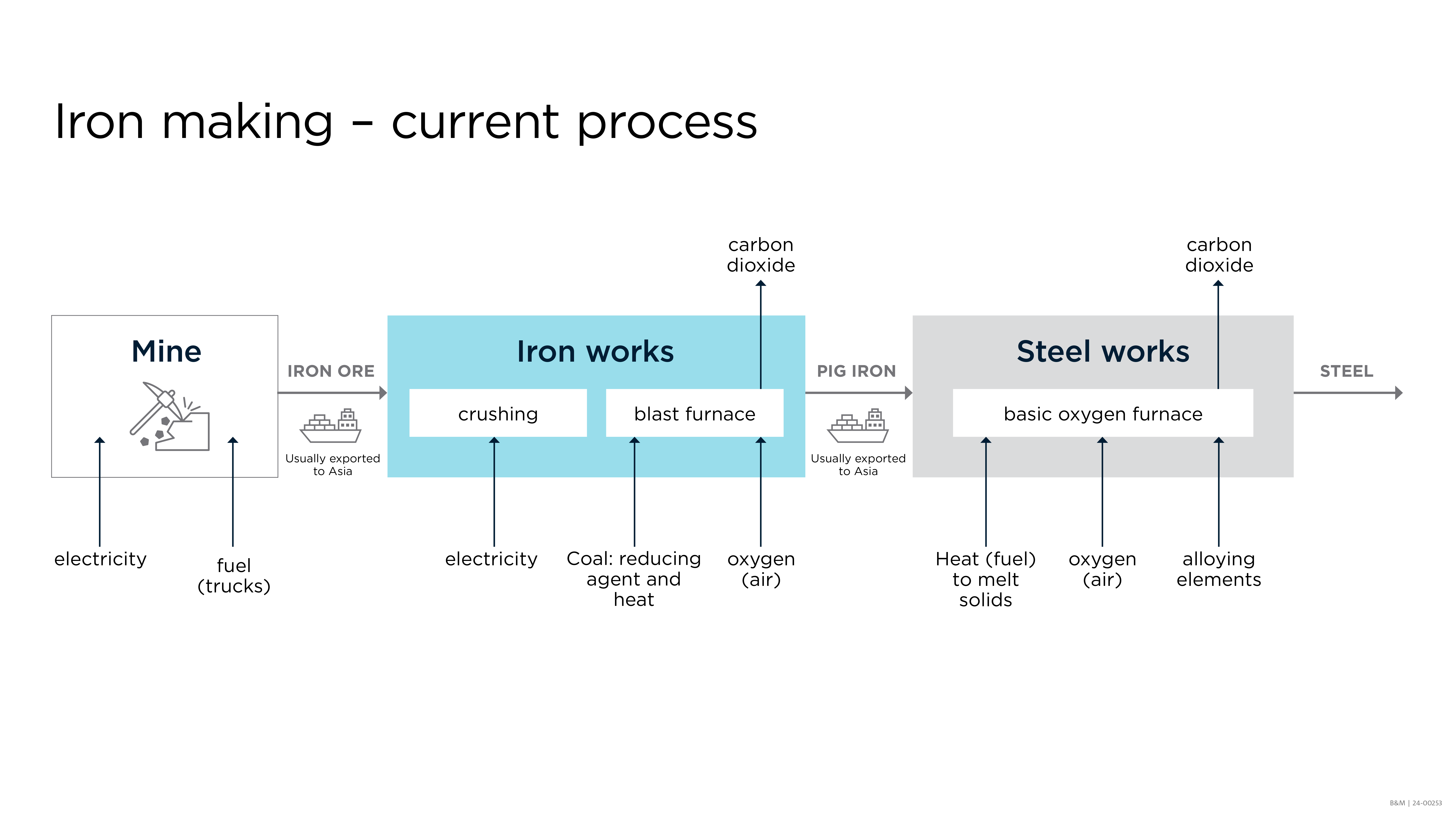
Once the iron ore is crushed, it goes into the blast furnace (either as lump ore or as agglomerates like sinters and pellets) where a reducing agent, usually a carbon-based material such as coal or coke (a special kind of coal) or natural gas, is burned to produce both heat and carbon monoxide. The carbon monoxide reacts with iron oxides (eg. Fe2O3 or Fe3O4) to produce iron and carbon dioxide; while the heat melts the iron to enable it to be removed from the furnace as a liquid. The carbon dioxide byproduct is a greenhouse gas.
How hydrogen can reduce greenhouse gas emissions
Some organisations are trialling the replacement of some of the coke with hydrogen in the blast furnace. Doing so reduces the amount of carbon dioxide produced.
A newer process, called Hydrogen Direct Reduced Iron (Hydrogen-DRI), uses hydrogen gas instead of carbon-based materials, such as coal or natural gas, to reduce the iron ore.
In traditional DRI methods, carbon-based materials are used to reduce iron ore in a reactor, producing a solid sponge-like form of iron called direct reduced iron. However, this process releases carbon dioxide similarly to the blast furnace method shown above.
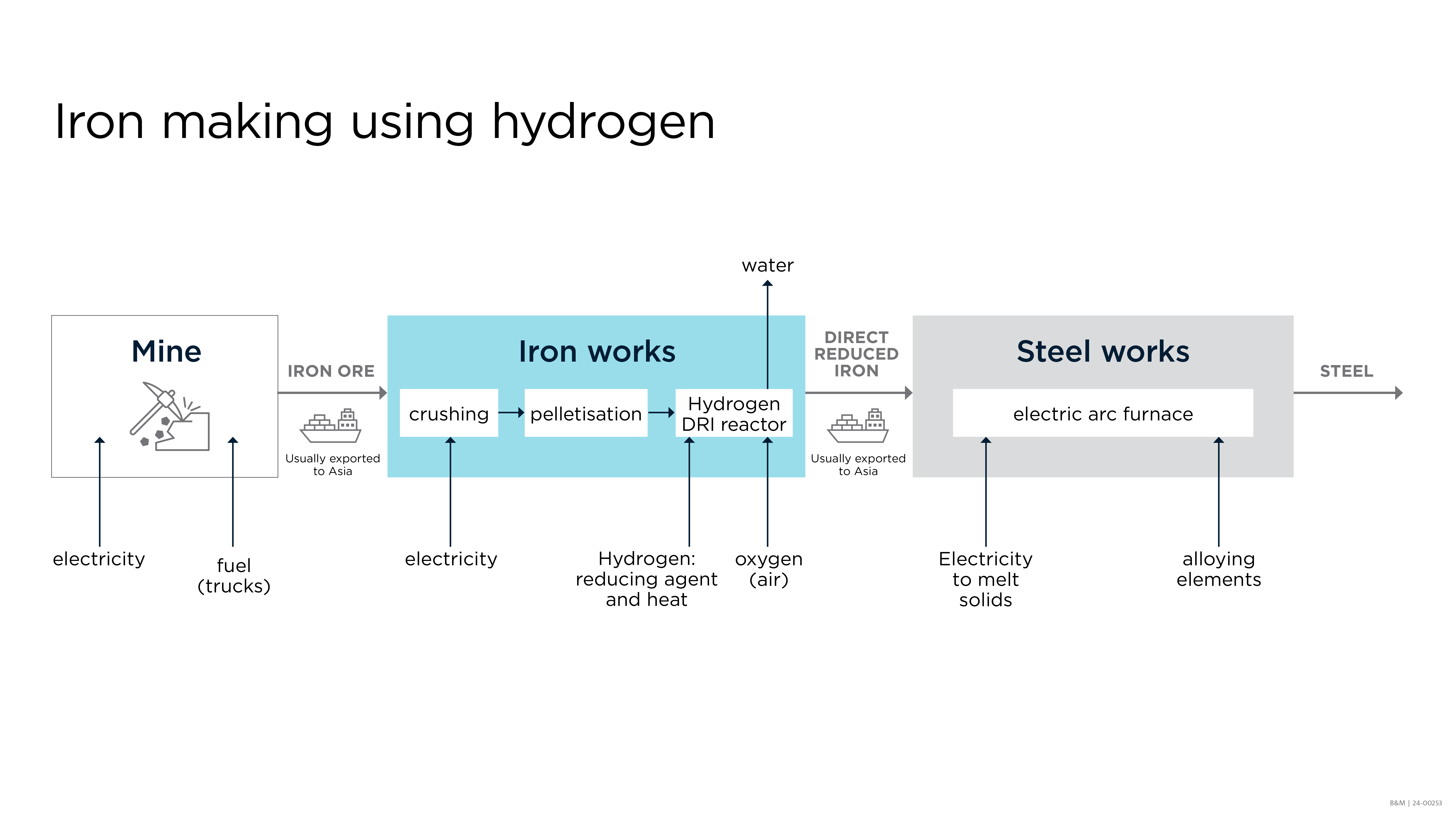
Hydrogen-DRI uses renewable hydrogen, which is produced from renewable energy sources such as wind or solar power, to reduce iron ore without producing carbon dioxide emissions. Some of the most promising Hydrogen-DRI routes such as HYBRIT and Energiron processes involve shaft-furnace type reactors and operate with pellets. These ironmaking processes involve crushing and agglomerating the iron ore, making small pellets. Those pellets are heated with hydrogen gas in a reactor, causing the oxygen in the ore to react with the hydrogen, producing water vapour and leaving behind pure iron.
Here is a simplified view of the process:
Challenges for Australia
The Hydrogen-DRI process works well with some iron ore types, such as magnetite, but it doesn’t work well with hematite and goethite, which are the most common types in the Pilbara, Australia’s biggest iron ore region. Pellets made from hematite and goethite are not as strong as magnetite pellets, so they can break up in the DRI reactor, and the alumina and silica sometimes found with those ores can cause sticking inside the reactor.
 The cost of renewable hydrogen is also currently a significant barrier. Renewable hydrogen is made by putting water through an electrolyser and applying electricity to split the water into hydrogen and oxygen. The cost of the electrolyser and current electricity costs combine to make renewable hydrogen a much more expensive heating and reducing agent than fossil fuels.
The cost of renewable hydrogen is also currently a significant barrier. Renewable hydrogen is made by putting water through an electrolyser and applying electricity to split the water into hydrogen and oxygen. The cost of the electrolyser and current electricity costs combine to make renewable hydrogen a much more expensive heating and reducing agent than fossil fuels.
A third challenge is that changing to the hydrogen processes, whether the blast furnace or the Hydrogen-DRI process, requires the construction of a new reactor. Iron makers are unlikely to replace their current reactors with new ones until they need to, and many of the reactors around the world are near the beginning of their multi-decade life.
While CSIRO can’t help with the age of the world’s reactor fleet, we are hard at work on research to both reduce the cost of hydrogen and improve the strength of hematite and goethite pellets.
What CSIRO is doing
CSIRO researchers are using our pilot-scale pelletisation facility to test different sintering and pelletisation techniques and then testing those pellets in a simulated furnace.
With funding from ARENA, CSIRO is working with Swinburne University of Technology, Grange Resources and Roy Hill to accelerate development of a novel iron ore agglomeration method designed to operate at lower temperature, while maintaining the desired physical and metallurgical agglomerate properties. The process has already been proven for Australia’s vastly underutilised magnetite concentrate and is currently being investigated for lower grade goethite rich iron ores from the Pilbara region.

We are also working with researchers in other countries. For example we are working with Indian researchers as part of the Australia-India Green Steel Partnership, to expedite the research happening in the green steel space and support the commercialisation of innovative Australian and Indian technology to improve efficiencies and environmental outcomes in steel production.
Meanwhile, we are researching a number of different methods for the production and storage of renewable hydrogen. In our electrolyser research, we are aiming to make electrolysers more efficient (so that they use less electricity) and more cost-efficient. And our research is having a real impact. For example, our electrolyser technology is licensed to Hadean Energy and we are trialling its use at the Bluescope steelworks in Port Kembla. Meanwhile, some of our other electrolyser technology is licensed to Endua who produce power banks for use off grid or in remote areas. Our Hydrogen Industry Mission’s goal is to support global decarbonisation through a commercially viable Australian hydrogen industry comprising both domestic and export value chains.
Please contact us if you would like to work with us on hydrogen research and development.
Other ways of reducing greenhouse gas emissions from the iron industry
While the carbon emissions from blast furnaces account for almost 90% of the iron making process, there are a number of other strategies for reducing the industry’s carbon footprint.
As shown in the diagrams above, electricity is used in several parts of the process. Using electricity from renewables such as solar and wind would greatly reduce the carbon emissions in electricity generation compared to traditional coal or gas fired power plants.
The iron ore and iron are transported around in trucks and ships. Replacing those vehicles with electric or hydrogen-powered vehicles would also reduce carbon emissions, as long as the electricity and/or hydrogen are produced renewably.
Much of our iron ore is transported overseas to be processed into pig iron. Given that iron ore contains about 50 to 70 wt.% Fe, we are using energy to transport a lot of extra material (mostly dirt and water) needlessly. If more iron ore was processed in Australia before exporting the pig iron, then much fewer ship journeys would be required, saving a lot of fuel.
Australia’s iron ore makes a significant contribution to our economy, with a gross added value of over $100 billion each year. However the process of turning the world’s iron ore into steel currently contributes around seven per cent of global greenhouse gas emissions. There are a number of ways of reducing the carbon footprint of the iron making industry, one of the most topical methods being the use of renewable hydrogen in the process.
How iron is made
Once iron ore is removed from the earth, it must be crushed into small pieces and then smelted to remove most of the oxygen, carbon and other impurities. The smelting process produces ‘pig iron’ which can be used to make cast iron, but most of it is converted into steel. Here is a simplified view of the current process, with inputs and outputs for each step:
The process flow diagram for the current standard iron making process shows the following steps:
Once the iron ore is crushed, it goes into the blast furnace (either as lump ore or as agglomerates like sinters and pellets) where a reducing agent, usually a carbon-based material such as coal or coke (a special kind of coal) or natural gas, is burned to produce both heat and carbon monoxide. The carbon monoxide reacts with iron oxides (eg. Fe2O3 or Fe3O4) to produce iron and carbon dioxide; while the heat melts the iron to enable it to be removed from the furnace as a liquid. The carbon dioxide byproduct is a greenhouse gas.
How hydrogen can reduce greenhouse gas emissions
Some organisations are trialling the replacement of some of the coke with hydrogen in the blast furnace. Doing so reduces the amount of carbon dioxide produced.
A newer process, called Hydrogen Direct Reduced Iron (Hydrogen-DRI), uses hydrogen gas instead of carbon-based materials, such as coal or natural gas, to reduce the iron ore.
In traditional DRI methods, carbon-based materials are used to reduce iron ore in a reactor, producing a solid sponge-like form of iron called direct reduced iron. However, this process releases carbon dioxide similarly to the blast furnace method shown above.
Hydrogen-DRI uses renewable hydrogen, which is produced from renewable energy sources such as wind or solar power, to reduce iron ore without producing carbon dioxide emissions. Some of the most promising Hydrogen-DRI routes such as HYBRIT and Energiron processes involve shaft-furnace type reactors and operate with pellets. These ironmaking processes involve crushing and agglomerating the iron ore, making small pellets. Those pellets are heated with hydrogen gas in a reactor, causing the oxygen in the ore to react with the hydrogen, producing water vapour and leaving behind pure iron.
Here is a simplified view of the process:
The process flow diagram for the current standard iron making process shows the following steps:
Challenges for Australia
The Hydrogen-DRI process works well with some iron ore types, such as magnetite, but it doesn’t work well with hematite and goethite, which are the most common types in the Pilbara, Australia’s biggest iron ore region. Pellets made from hematite and goethite are not as strong as magnetite pellets, so they can break up in the DRI reactor, and the alumina and silica sometimes found with those ores can cause sticking inside the reactor.
The cost of renewable hydrogen is also currently a significant barrier. Renewable hydrogen is made by putting water through an electrolyser and applying electricity to split the water into hydrogen and oxygen. The cost of the electrolyser and current electricity costs combine to make renewable hydrogen a much more expensive heating and reducing agent than fossil fuels.
A third challenge is that changing to the hydrogen processes, whether the blast furnace or the Hydrogen-DRI process, requires the construction of a new reactor. Iron makers are unlikely to replace their current reactors with new ones until they need to, and many of the reactors around the world are near the beginning of their multi-decade life.
While CSIRO can’t help with the age of the world’s reactor fleet, we are hard at work on research to both reduce the cost of hydrogen and improve the strength of hematite and goethite pellets.
What CSIRO is doing
CSIRO researchers are using our pilot-scale pelletisation facility to test different sintering and pelletisation techniques and then testing those pellets in a simulated furnace.
With funding from ARENA, CSIRO is working with Swinburne University of Technology, Grange Resources and Roy Hill to accelerate development of a novel iron ore agglomeration method designed to operate at lower temperature, while maintaining the desired physical and metallurgical agglomerate properties. The process has already been proven for Australia’s vastly underutilised magnetite concentrate and is currently being investigated for lower grade goethite rich iron ores from the Pilbara region.
We are studying the kinetic mechanisms of iron reduction using hydrogen, and the potential use of ammonia (a derivative of hydrogen) as a reductant in iron making.
We are also working with researchers in other countries. For example we are working with Indian researchers as part of the Australia-India Green Steel Partnership, to expedite the research happening in the green steel space and support the commercialisation of innovative Australian and Indian technology to improve efficiencies and environmental outcomes in steel production.
Meanwhile, we are researching a number of different methods for the production and storage of renewable hydrogen. In our electrolyser research, we are aiming to make electrolysers more efficient (so that they use less electricity) and more cost-efficient. And our research is having a real impact. For example, our electrolyser technology is licensed to Hadean Energy and we are trialling its use at the Bluescope steelworks in Port Kembla. Meanwhile, some of our other electrolyser technology is licensed to Endua who produce power banks for use off grid or in remote areas. Our Hydrogen Industry Mission’s goal is to support global decarbonisation through a commercially viable Australian hydrogen industry comprising both domestic and export value chains.
Please contact us if you would like to work with us on hydrogen research and development.
Other ways of reducing greenhouse gas emissions from the iron industry
While the carbon emissions from blast furnaces account for almost 90% of the iron making process, there are a number of other strategies for reducing the industry’s carbon footprint.
As shown in the diagrams above, electricity is used in several parts of the process. Using electricity from renewables such as solar and wind would greatly reduce the carbon emissions in electricity generation compared to traditional coal or gas fired power plants.
The iron ore and iron are transported around in trucks and ships. Replacing those vehicles with electric or hydrogen-powered vehicles would also reduce carbon emissions, as long as the electricity and/or hydrogen are produced renewably.
Much of our iron ore is transported overseas to be processed into pig iron. Given that iron ore contains about 50 to 70 wt.% Fe, we are using energy to transport a lot of extra material (mostly dirt and water) needlessly. If more iron ore was processed in Australia before exporting the pig iron, then much fewer ship journeys would be required, saving a lot of fuel.
