CSIRO’s Biomedical Manufacturing Program are working closely with industry and Minimising AMR to find solutions to the threat of AMR across three key areas:
- Therapeutics
- Medical devices
- Vaccines.
We provide R&D and multidisciplinary expertise to industry, from start-ups to large enterprises, both nationally and internationally, to develop and apply new technologies, products and processes, and help grow the AMR industry ecosystem.
Our advanced manufacturing capabilities can help you translate your ideas into market-ready products.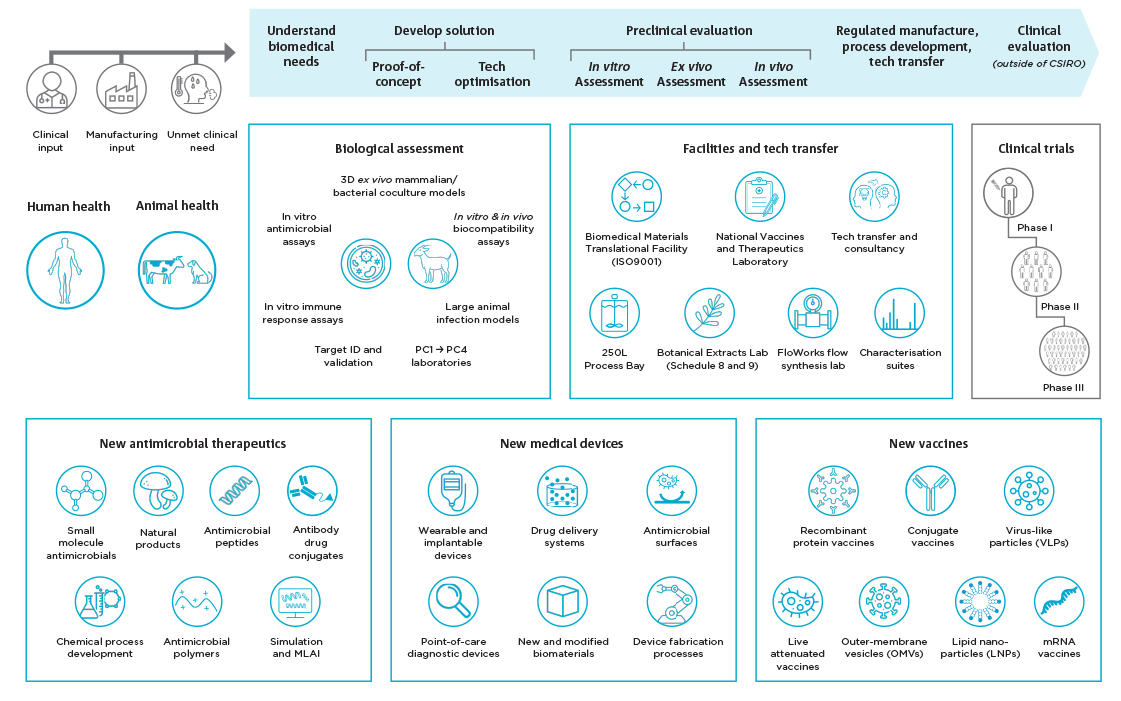
Facilities to support AMR research
As one of the world’s largest multi‑disciplinary science organisations, CSIRO has world‑class facilities and expertise to support AMR research, such as:
- National Vaccines and Therapeutics Laboratory (NVTL)
- Biomedical Materials Translational Facility (BMTF)
- Centre for Industrial Flow Chemistry (FloWorks)
- Botanical Extracts Laboratory (BEL)
- Rapid Automated Materials and Processing (RAMP)
How can we assist industry?
1. Drug discovery and bioactive compounds
- Structure-based drug discovery.
- Hit identification, hit-to-lead, and lead optimisation.
- Novel compound libraries that act as starting points, such as the CSIRO Compound Collection.
- Growing capabilities in development of AI tools and simulation to aid drug discovery.
- Peptide chemistry.
- Bioactive natural products.
- Polymer mimetics of antimicrobial peptides.
- Bespoke assay development to assist discovery.
2. Biocompatible materials for medical applications
- Design of novel polymers and materials for medical devices and drug delivery.
- Surface chemistry and surface modification.
- Design and fabrication of biosensors and diagnostic devices.
- Biological assessment of materials and devices.
3. Production solutions
- Production of proteins and vaccines for:
- humans (clinical trials)
- animals (veterinary clinical trials).
- Production of proteins for assay development and diagnostic devices.
- Scale up synthesis for small molecules through both batch and flow processes.
- Development of bespoke extraction processes for isolation of bioactive natural products.
- Development of medical device fabrication processes, including extrusion, moulding, coating, and 3D printing.
- Regulated laboratories for translation of various technologies into clinical applications.
4. Funding identification
CSIRO’s in-house Kick Start grants facilitated by CSIRO Science Connect, support eligible SMEs with matched non-dilutive funding.
- Pending eligibility, funding may also be available from Therapeutic Innovation Australia (TIA):
- TIA Pipeline Accelerator voucher scheme
- Separate TIA subsidies to support research at CSIRO.
We are happy to work with industry to identify any relevant funding opportunities to support their research with CSIRO, suited to their individual situation.
For more information, please contact the AMR Technologies Market Area co-leads:
CSIRO’s Biomedical Manufacturing Program are working closely with industry and Minimising AMR to find solutions to the threat of AMR across three key areas:
- Therapeutics
- Medical devices
- Vaccines.
We provide R&D and multidisciplinary expertise to industry, from start-ups to large enterprises, both nationally and internationally, to develop and apply new technologies, products and processes, and help grow the AMR industry ecosystem.
Our advanced manufacturing capabilities can help you translate your ideas into market-ready products.
Across the top of this diagram: Outlining the process of solution development Within human and animal health: combine these factors: Before undertaking these steps: Below this flow chart are boxes focuses on capability areas and features: Biological assessment Facilities and tech transfer Clinical trials (outside of CSIRO) Phase 1 through 3 As well as capabilities in:
Tech transfer and consultancy
Facilities to support AMR research
As one of the world’s largest multi‑disciplinary science organisations, CSIRO has world‑class facilities and expertise to support AMR research, such as:
- National Vaccines and Therapeutics Laboratory (NVTL)
- Biomedical Materials Translational Facility (BMTF)
- Centre for Industrial Flow Chemistry (FloWorks)
- Botanical Extracts Laboratory (BEL)
- Rapid Automated Materials and Processing (RAMP)
How can we assist industry?
1. Drug discovery and bioactive compounds
- Structure-based drug discovery.
- Hit identification, hit-to-lead, and lead optimisation.
- Novel compound libraries that act as starting points, such as the CSIRO Compound Collection.
- Growing capabilities in development of AI tools and simulation to aid drug discovery.
- Peptide chemistry.
- Bioactive natural products.
- Polymer mimetics of antimicrobial peptides.
- Bespoke assay development to assist discovery.
2. Biocompatible materials for medical applications
- Design of novel polymers and materials for medical devices and drug delivery.
- Surface chemistry and surface modification.
- Design and fabrication of biosensors and diagnostic devices.
- Biological assessment of materials and devices.
3. Production solutions
- Production of proteins and vaccines for:
- humans (clinical trials)
- animals (veterinary clinical trials).
- Production of proteins for assay development and diagnostic devices.
- Scale up synthesis for small molecules through both batch and flow processes.
- Development of bespoke extraction processes for isolation of bioactive natural products.
- Development of medical device fabrication processes, including extrusion, moulding, coating, and 3D printing.
- Regulated laboratories for translation of various technologies into clinical applications.
4. Funding identification
CSIRO’s in-house Kick Start grants facilitated by CSIRO Science Connect, support eligible SMEs with matched non-dilutive funding.
- Pending eligibility, funding may also be available from Therapeutic Innovation Australia (TIA):
- TIA Pipeline Accelerator voucher scheme
- Separate TIA subsidies to support research at CSIRO.
We are happy to work with industry to identify any relevant funding opportunities to support their research with CSIRO, suited to their individual situation.
For more information, please contact the AMR Technologies Market Area co-leads:
